Compliance Consultants has for years worked
for one of the world's largest women's health /
OBGYN companies who only produced
women's health products.
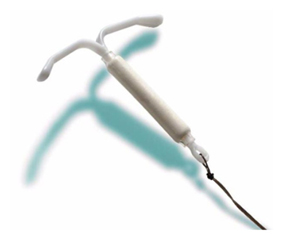
Compliance Consultants has worked on two forms of birth control: female (reversalable) sterilization - Hulka / Wolfe clip that temporarily blocks the fallopian tubes. The Hulka / Wolfe clip is applied under Laparoscopic surgery. It is accepted as the most reversible form of female sterilization. The other is the intrauterine device (IUD) is a form of birth control; it is an object placed in the uterus to prevent pregnancy.
Regulation Number 876.4020, Product Code FCS, Transilluminating Ureteric Stent, and Ureteral Fiberoptic Catheter
Regulation Number 21 CFR 884.1175, Product Code: HHK, Endometrial Sampling Syringe.
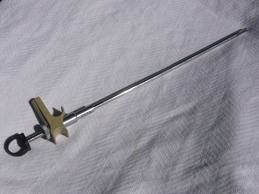
Compliance Consultants has worked on two forms of birth control: female (reversalable) sterilization - Hulka / Wolfe clip that temporarily blocks the fallopian tubes. The Hulka / Wolfe clip is applied under Laparoscopic surgery. It is accepted as the most reversible form of female sterilization. The other is the intrauterine device (IUD) is a form of birth control; it is an object placed in the uterus to prevent pregnancy.
Regulation Number 876.4020, Product Code FCS, Transilluminating Ureteric Stent, and Ureteral Fiberoptic Catheter
Regulation Number 21 CFR 884.1175, Product Code: HHK, Endometrial Sampling Syringe.


