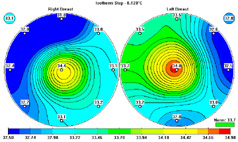
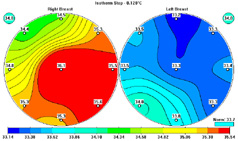
As part of the extensive backgrounds that Compliance
Consultants has in OB / GYN medical devices, is that
we worked with pioneers to produce low cost, painless,
convenient mammographic alternatives to low dose
radiation. Conventional mammography is known to
return many false positives. Women in the highest risk
groups or young women (where the worst outcomes
occur) - do not have scans on the most frequent basis.
This infrared mammography screening tool offers an
alternative.
Infrared mammography, thermography imaging system. Regulation Number: 21 CFR 884.2980, Product Code- LHQ, IYM, LHQ
Regulation Number: 21 CFR 884.2982 Product Code- IYM
Regulation Number: 21 CFR 884.2980, Product Code: LHQ
Telethermographic Systems Mammography Thermography, OB /GYN
Regulation Number: 21 CFR 884.2982, Product Code: IYM, Liquid Crystal Infrared Frequency Thermography System
Infrared mammography, thermography imaging system. Regulation Number: 21 CFR 884.2980, Product Code- LHQ, IYM, LHQ
Regulation Number: 21 CFR 884.2982 Product Code- IYM
Regulation Number: 21 CFR 884.2980, Product Code: LHQ
Telethermographic Systems Mammography Thermography, OB /GYN
Regulation Number: 21 CFR 884.2982, Product Code: IYM, Liquid Crystal Infrared Frequency Thermography System

