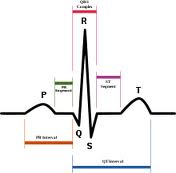
Compliance Consultants has produced applications for almost every
variety of ECG monitors: lab, clinical, portable, 1, 3 to 12 leads. This
includes: lead wires and their electrodes. These Class II devices
measure and display EGC waveforms, R-R graph, an average heart
rate for acquisition and transmission of ECG data to multiple sources.
Compliance Consultants submitted one of the first computerized electrophysiology amplifier systems. This system used off the shelf computer that interfaced to a single ended A/D to detect, record and display cardiac arrhythmias from patients using standard electrodes. These systems use cardiac electro physiology amplifiers that collect monomorphic atrial and ventricular arrhythmias from patients in real- time. One example is an invasive basket catheter that is inserted in the leg and runs into the beating heart and expands on contact the interior surface of the heart. Data collected is displayed, recorded and transmitted in real-time positional information for analysis.
Many are tested to harmonized standard: EC 38
Regulation Number: 21 CFR 870.2800, Product Code: DSH
Regulation Number: 21 CFR 870.2050, Product Code: DRR
Guidance for Industry and FDA Staff Class II Special Controls Guidance Document: Arrhythmia Detector and Alarm. This specifies controls to reclassify arrhythmia detector and alarm into class II (special controls). The device is intended to monitor an electrocardiogram (ECG) 1 and to produce a visible or audible signal or alarm when an atrial or ventricular arrhythmia exists. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/ GuidanceDocuments/ucm072673.pdf
Application of the test voltage between LEAD WIRES to test the energy delivered. ANSI/AAMI EC 38 Medical electrical equipment, Part 2-47: Particular requirements, for the safety, including essential performance, of ambulatory electrocardiographic systems, minimum labeling, performance, and safety requirements. [marketplace.aami.org/]
Compliance Consultants submitted one of the first computerized electrophysiology amplifier systems. This system used off the shelf computer that interfaced to a single ended A/D to detect, record and display cardiac arrhythmias from patients using standard electrodes. These systems use cardiac electro physiology amplifiers that collect monomorphic atrial and ventricular arrhythmias from patients in real- time. One example is an invasive basket catheter that is inserted in the leg and runs into the beating heart and expands on contact the interior surface of the heart. Data collected is displayed, recorded and transmitted in real-time positional information for analysis.
Many are tested to harmonized standard: EC 38
Regulation Number: 21 CFR 870.2800, Product Code: DSH
Regulation Number: 21 CFR 870.2050, Product Code: DRR
Guidance for Industry and FDA Staff Class II Special Controls Guidance Document: Arrhythmia Detector and Alarm. This specifies controls to reclassify arrhythmia detector and alarm into class II (special controls). The device is intended to monitor an electrocardiogram (ECG) 1 and to produce a visible or audible signal or alarm when an atrial or ventricular arrhythmia exists. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/ GuidanceDocuments/ucm072673.pdf
Application of the test voltage between LEAD WIRES to test the energy delivered. ANSI/AAMI EC 38 Medical electrical equipment, Part 2-47: Particular requirements, for the safety, including essential performance, of ambulatory electrocardiographic systems, minimum labeling, performance, and safety requirements. [marketplace.aami.org/]