The FDA will require tests concerning adverse tissue reactions to the skin- contacting electrode materials: Dermal irritation/delayed sensitivity: ISO 10993-10, Cytotoxicity: ISO 10993-5.
Compliance Consultants can design, test and submit 510(k)
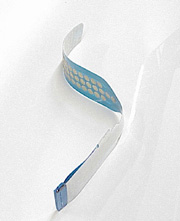
applications for advanced multiple hydrogel electrodes for ECG
and TENS applications.
We can develop biocompatibility, electrical, adhesive and shelf life performance data. These devices include bare metal ECG electrodes or ECG electrodes that incorporate, as part of their design, a conductive gel, an adhesive system, or a lead-wire. ECG electrodes may be supplied for use as reusable, disposable, sterile, or non-sterile products. These electrodes or dispersive pads can be used to deliver therapy such as defibrillation or receive signals such as ECG. Electrodes or dispersive pads used to deliver defibrillation or for cardioversion are regulated under 21 CFR 870.5310.
The scope excludes electrodes used for external pacing or other treatment effect. Electrodes used for external pacing are regulated under 21 CFR 870.5550. Material & construction such as bare metal, conductive gel, adhesive system and lead wires whether they are reusable, disposable, sterile or non-sterile and the packaging and labeling are of concern to CDRH.
We can develop biocompatibility, electrical, adhesive and shelf life performance data. These devices include bare metal ECG electrodes or ECG electrodes that incorporate, as part of their design, a conductive gel, an adhesive system, or a lead-wire. ECG electrodes may be supplied for use as reusable, disposable, sterile, or non-sterile products. These electrodes or dispersive pads can be used to deliver therapy such as defibrillation or receive signals such as ECG. Electrodes or dispersive pads used to deliver defibrillation or for cardioversion are regulated under 21 CFR 870.5310.
The scope excludes electrodes used for external pacing or other treatment effect. Electrodes used for external pacing are regulated under 21 CFR 870.5550. Material & construction such as bare metal, conductive gel, adhesive system and lead wires whether they are reusable, disposable, sterile or non-sterile and the packaging and labeling are of concern to CDRH.

