Compliance Consultants can submit your
balloon catheter for a 510(k). We can operate
from the early onset of product development to
the marketing release. Percutaneous
Transluminal Angioplasty (PTA) Catheter
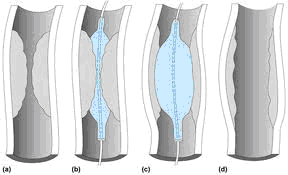
can be performed to treat vessel narrowing. A
wire is passed from the femoral artery in the
groin (or, at times, from the radial artery or
brachial artery in the arm) to beyond the area of
the artery that is being treated. A balloon
catheter is advanced over the wire to the
segment that is to be treated. The end of the
catheter contains a small folded balloon. When
the balloon is inflated, it compresses the plaque
and stretches the artery wall to expand,
improving blood flow. Regulation Number 21
CFR 870.1250, Product Code LIT and DQY.