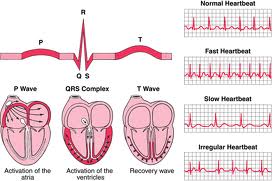
Compliance Consultants worked on cardiographic catheters
and mapping systems that map the interteior surfaces of
beating heart. Maps the electrical activity of interior heart
surfaces are displayed as 3D data using computer diagnostic
software. Used in monomorphic, stationary atrial and
ventricular arrhythmias. This device detects and displays
cardiac depolarization events from acquired
electrophysiological data.
Regulation Number: 21 CFR 870.1425, Product Code: DQK and Regulation Number: 21 CFR 870.1875, Product Code: DQD.
Regulation Number: 21 CFR 870.1425, Product Code: DQK and Regulation Number: 21 CFR 870.1875, Product Code: DQD.

