Off-Label use of Medical Devices
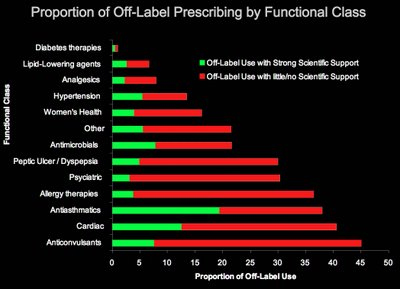
We have had much experience in the requirements for "off-label" use of non-significant risk, Class II devices. This has always been "convoluted territory". The FDA / CDRH has issued some guidelines for the marketing statements that a medical device manufacturer can make regarding "off-label" use."Off label prescriptions" are written by the millions each week by medical doctors in the US without thought! Look at the "Indications for Use" on any bottle of aspirin. Aspirin used to thin the blood is prescribed "off label" routinely by physicians with no hesitation. Why are pharmaceutical preparations given a privilege over a harmless medical device? Is that really science or medical convention?
Off label prescription are written by the millions each week in the US by medical doctors. Why is a physician reluctant to write and off label prescription for a harmless medical device?
Off label prescriptions account for almost 60% of all the prescriptions written in America yet seldom what you see doctors prescribe medical devices "off label" Why is this. Even though the deaths from pharmaceutical preparations now exceed the number of people in America that are killed by automobile accidents. It appears that pharmaceuticals are considered safer than medical devices.
Good Reprint Practices for the Distribution of Medical Journal Articles and Medical or Scientific Reference Publications on Unapproved New Uses of Approved Drugs and Approved or Cleared Medical Devices. http://www.fda.gov/RegulatoryInformation/Guidances/ucm125126.htm