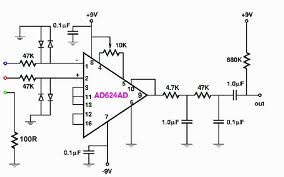
Compliance Consultants can develop medical
electronic circuits on your requirements and
organize system controls to meet FDA / CDRH
requirements for a broad range of applications.
Compliance Consultants can design circuits to IEC 60601 for: stepper motors, laser drivers, process control systems, etc. Whatever the medical device, we can take it from proof of concept through prototype, pre-production, test rationale. test procedures and life cycle planning.
If you have issues such as: isolation, noise, delays, etc. Ask us, it is likely we have faced these before.
We can identify the harmonized standards and test to those standards. We can create and manage compliance testing & qualification to UL, EC, ISO, 60601 and numerous other standards.
Compliance Consultants can design circuits to IEC 60601 for: stepper motors, laser drivers, process control systems, etc. Whatever the medical device, we can take it from proof of concept through prototype, pre-production, test rationale. test procedures and life cycle planning.
If you have issues such as: isolation, noise, delays, etc. Ask us, it is likely we have faced these before.
We can identify the harmonized standards and test to those standards. We can create and manage compliance testing & qualification to UL, EC, ISO, 60601 and numerous other standards.