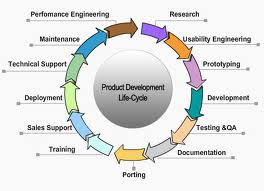
A new trend is emerging where CDRH requirements now
focus on adapting a life cycle approach to a determination of
substantial equivalence for medical devices. This cycle runs
from inception to product "end of life". Typically, in a high
velocity marketplace (such as medical device software) this
period can be a mere six years.
Compliance Consultants understands conceptual medical product development of either hardware or software. We can work from conceptual designs on a napkins to the other end of the spectrum such as retiring medical devices in the field. Ask us- we cover the entire range. Some Harmonized Standards such as ANSI/AAMI SW 68:2001, Medical Device software may apply.
Compliance Consultants understands conceptual medical product development of either hardware or software. We can work from conceptual designs on a napkins to the other end of the spectrum such as retiring medical devices in the field. Ask us- we cover the entire range. Some Harmonized Standards such as ANSI/AAMI SW 68:2001, Medical Device software may apply.

